COVID-19 Vaccine Trial
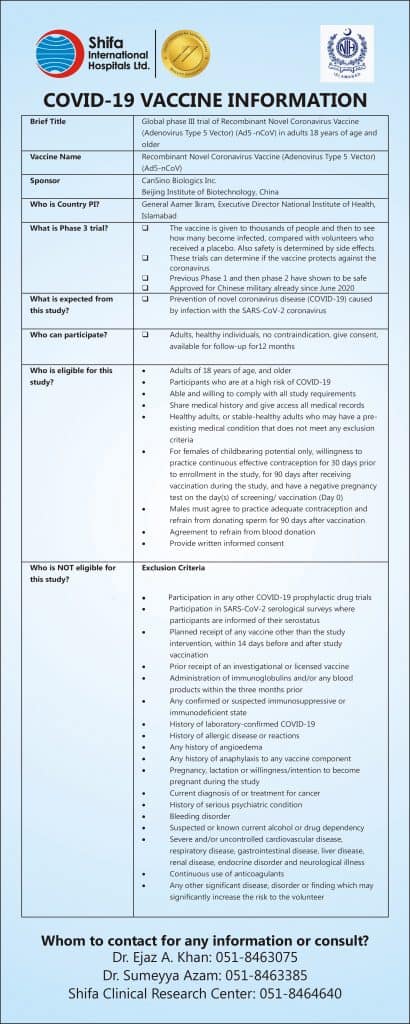
Shifa International Hospital has started the trial of the COVID-19 vaccine. We invite volunteers to be part of this global multicenter, randomized, double-blind, placebo-controlled, adaptive designed Phase III clinical trial.
The vaccine is named Recombinant Novel Coronavirus Vaccine Adenovirus Type 5 Vector (Ad5-nCoV) and has been developed by CanSinoBio and Beijing Institute of Biotechnology China. Shifa International Hospital is collaborating with the National Institute of Health Pakistan and CanSino Biologics, Inc. China for these trials along with four other centers in Pakistan.
Pakistan is among 7 countries that will carry out the Phase III trial in compliance with National and International Ethical & Regulatory Guidelines. Shifa International Hospital is the first site that launched this trial in Pakistan and the first batch of participants in Pakistan has been recruited.
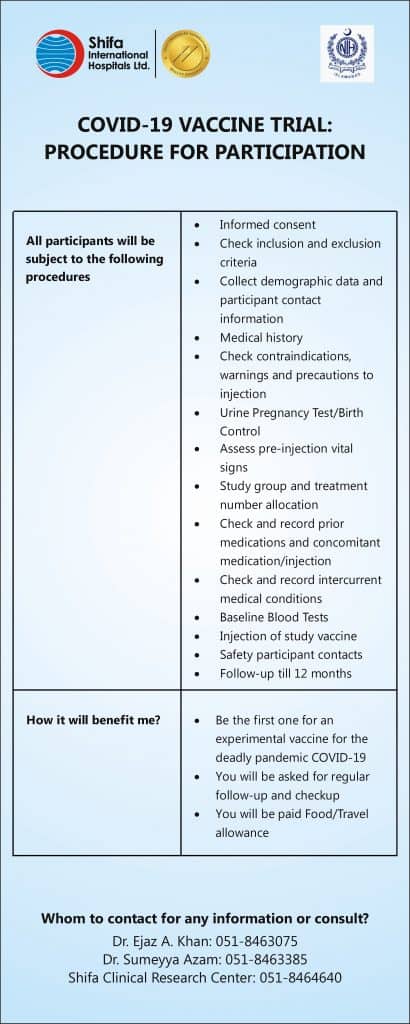
Shifa International Hospital invites volunteers from your institute to be part of this Global Landmark Vaccination trial. This clinical trial will evaluate the efficacy, safety, and immunogenicity of this vaccine. This vaccine has shown to be successful in Phase I and Phase II.
Your cooperation is important for us to fight this COVID-19 pandemic. Interested volunteers can feel free to contact at 051-8464729 / 30 for registration and/ or any further information.

https://www.bbc.com/urdu/pakistan-54298495
https://www.arabnews.pk/node/1742331/pakistan
https://www.youtube.com/embed/zsq9r3Os5YQ
https://www.dawn.com/news/1582552/doctor-urges-people-to-volunteer-for-covid-vaccine-trial?preview
https://www.geo.tv/latest/310795-people-in-pakistan-urged-to-volunteer-for-covid-19-vaccine-trial
ATV (Morning Show)
https://www.youtube.com/watch?v=s-ITGnrHIps&feature=youtu.be
92 News (Morning Show)
https://www.youtube.com/watch?v=4bmbv_uuZc8
Roze News (Roze Clinic)
https://www.youtube.com/watch?v=2Kbqi9zO7ss
PTV World (Perspective)
https://www.youtube.com/watch?v=lVRhvdoI614&feature=youtu.be
Such TV (Sehat Zindagi)
https://www.suchtv.pk/program/sehat-zindagi/item/102310-sehat-zindagi-19-11-2020.html
DAWN
https://www.dawn.com/news/1592651
Samaa TV
https://www.samaa.tv/news/2020/11/pakistanis-join-final-trials-for-china-made-covid-19-vaccine/
Express Tribune
https://tribune.com.pk/story/2273670/pakistanis-join-final-trials-for-china-made-vaccine?amp=1
Geo News
https://www.geo.tv/latest/320780-pakistanis-join-final-trials-for-china-made-coronavirus-vaccine
Urdu News
https://www.urdunews.com/node/520901