- Home
- Clinical Trial Unit
- Clinical & Research Trials
- Cancer Registry
- Publication
- Useful Links
- Team
- News & Events
- Editorials
- Testimonials
Shifa Clinical Research Center
Vision
To be a regional leader in innovative and groundbreaking clinical research, to advance medical knowledge (“Illaman Nafian”), and to improve the well-being of the community at large by fostering collaboration among researchers, clinicians, and industry partners.
Mission
To provide an unparalleled platform in the region, uniquely positioned to lead impactful, and ethical clinical research that transforms clinical practice on a global scale.
Message from the Director
Welcome to the Clinical Research Centre, where innovation, ethics, and dedication drive our mission to advance healthcare. We are committed to conducting groundbreaking research that not only transforms lives but does so with the utmost integrity and respect for the individuals and communities we serve.
Through collaborative efforts with researchers, healthcare professionals, and industry leaders, we strive to pioneer solutions to the world’s most pressing medical challenges. Embracing cutting-edge technologies like artificial intelligence, we aim to enhance precision, accelerate timelines, and uphold the highest ethical standards in clinical trials, ensuring that our work remains patient-centered and socially responsible.
Prof. Dr. Maimoona Siddiqui
Director, Shifa Clinical Research Center & Medical Education
Professor of Neurology, Shifa College of Medicine, Shifa Tameer-e-Millat University, Islamabad
Contact Information
Shifa Clinical Research Center, Block H-3 Shifa International Hospitals Ltd.
Pitras Bukhari Road H-8/4, Islamabad – Pakistan
Telephone:
+92 – 51–8463984
+92 – 51–8463985
+92 – 51–8463385
+92 – 51–8464857
Email: scrc@shifa.com.pk
Shifa Clinical Research Center
Main Office A-0
Inpatient Unit
Research Reception A-0
Research Phlebotomy A-0
Clinical Trial Unit (CTU) A-0
Clinical Trial Repository (CTR) I-9
CTU Clinic
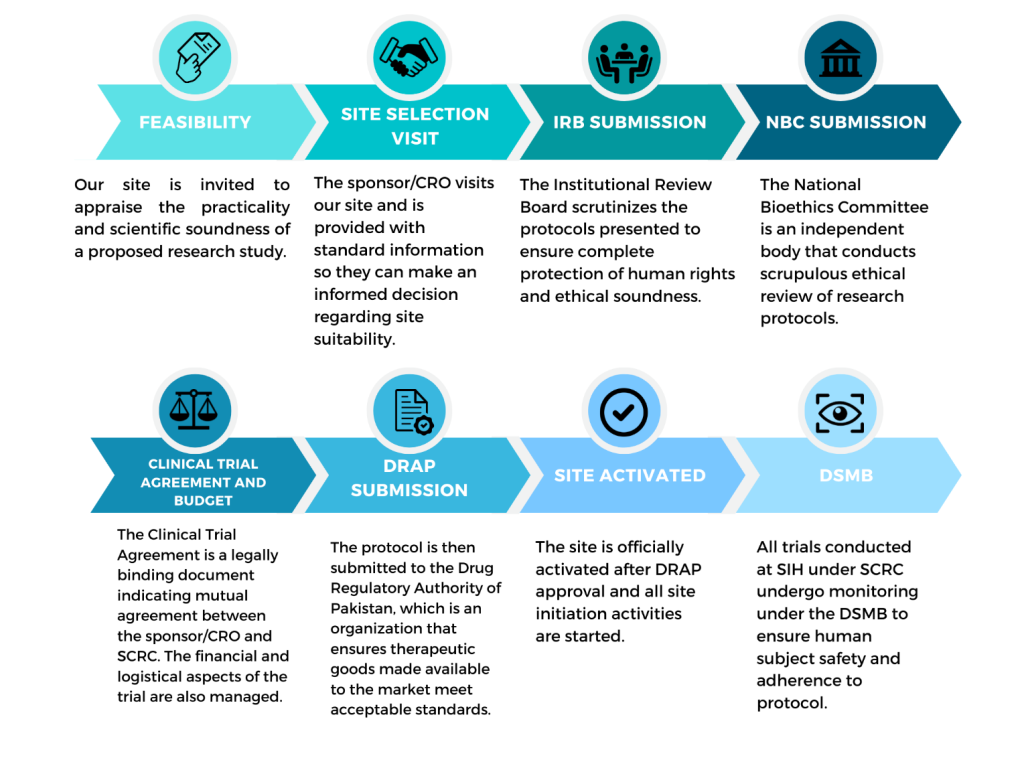
CLINICAL TRIAL APPROVAL PROCESS
Shifa Hospital Research Trials
| TRIALS NAMES | SPONSORS | STATUS |
|
HALT-IT Hemorrhage alleviation with tranexamic acid-intestinal system, Tranexamic acid of the treatment of gastrointestinal bleeding: An International randomized, double-blind placebo-controlled trial. |
 |
Completed Published in Trials https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3561-7 |
|
CRASH-3 Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomized, placebo-controlled trial |
 |
Completed Published in The Lancet https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32233-0/fulltext |
|
HIP ATTACK 1 Accelerated surgery versus standard care in hip fracture an international, randomized, controlled trial |
Completed Published in The Lancet https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30058-1/fulltext |
|
|
SafeHer Study A phase-III Prospective, two-cohort non-randomized Multicenter, Multinational, open-label study to assess the safety of assisted- and self-administered subcutaneous Trastuzumab as therapy in patients with operable her2-positive early breast cancer |
 |
Completed
https://academic.oup.com/oncolo/article/23/10/1137/6439744
|
|
PERUSE Study A phase III prospective, two-cohort non-randomized, Multicenter, Multinational, open-label single arm study of pertuzumab in combination of as THERAPY in patients with operable Her2-positive early breast cancer |
 |
Completed |
|
POISE-3 Perioperative Ischemic Evaluation-3 |
Completed Published in PubMed, NEJM https://pubmed.ncbi.nlm.nih.gov/35101083/
https://www.nejm.org/doi/full/10.1056/NEJMoa2201171 |
|
|
Can Sino Phase III Trial of A COVID-19 Vaccine of Adenovirus Vector in Adults 18 Years Old and Above |
 |
Completed Published in The Lancet https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02753-7/fulltext |
|
COP-AF Colchicine For The Prevention Of Perioperative Atrial Fibrillation In Patients Undergoing Thoracic Surgery |
|
Completed Published in The Lancet, PubMed, European Society of Cardiology
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01689-6/fulltext
https://pubmed.ncbi.nlm.nih.gov/37640035/
https://www.escardio.org/The-ESC/Press-Office/Press-releases/Colchicine-fails-to-reduce- |
|
Livzon Global Phase 3 Randomized Trial to evaluate efficacy, safety, immunogenicity of Recombinant SARS COV-2 fusion protein vaccine against COVID 19 in healthy adults after vaccination of 2 doses of inactivated vaccines |
 |
Completed Published in Emerging Microbes & Infections https://www.tandfonline.com/doi/full/10.1080/22221751.2022.2088406 |
|
DEPOSITION Decreasing Postoperative Blood Loss by Topical vs. Intravenous Tranexamic Acid in Open Cardiac Surgery |
 |
Completed |
|
SEQIRUS A Phase III, Randomized, Observer-blind, Multicenter Study to Evaluate the Efficacy, Immunogenicity and Safety of Seqirus’ Cell-Based Quadrivalent Subunit Influenza Virus Vaccine (QIVc) Compared to a Non-Influenza Vaccine when Administrated in Healthy Subjects aged 6 Months through 47 Months
|
 |
Completed https://www.clinicaltrials.gov/ct2/show/NCT03165617 |
|
BCD-201 A Randomized, Double-Blind Clinical Study of the Efficacy and Safety of BCD-201(JSC BIOCAD) and Keytruda® in Patients with Unresectable or Metastatic Melanoma |
 |
Completed |
|
GATES-MRI-301 A Phase 3, randomized, double-blind, placebo-controlled study to evaluate the effect of Bi-26 (strain ofBifidobacterium longum, B. infantis) supplementation versus placebo on weight gain in underweight infants |
 |
Completed |
|
ALVOEYE A Randomized, Double-masked, Parallel-group, MulticenterClinical Study to Evaluate the Efficacy and Safety of AVT06Compared with EU-Eylea® in Subjects with Neovascular (wet)Age-related Macular Degeneration |
 |
Completed |
|
ASPIRE-AF Anticoagulation for Stroke Prevention In patients with Recent Episodes of perioperative Atrial Fibrillation after noncardiac surgery |
 |
In Progress https://clinicaltrials.gov/ct2/show/NCT03968393
|
|
BCD-178-2 A Double-Blind, Randomized Clinical Study of the Efficacy and Safety of BCD-178 and Perjeta® as Neoadjuvant Therapy of HER2-Positive Breast Cancer
|
 |
In Progress |
|
HIP ATTACK-2 Accelerated surgery versus standard care in hip fracture an international, randomized, controlled trial
|
 |
In Progress https://clinicaltrials.gov/ct2/show/NCT04743765
|
|
DARVIVA/ BCD-264-2 A Double-Blind, Randomized Clinical Study of the Efficacy and Safety of Monotherapy with BCD-264 and Darzalex® in Subjects with Relapsed and Refractory Multiple Myeloma |
 |
Actively recruiting https://clinicaltrials.gov/study/NCT06296121?id=NCT06296121&rank=1 |
|
LIMIT To assess the feasibility of a large trial to evaluate the safety and efficacy of a common, lower INR target range in patients with bileaflet aortic mechanical valves. |
 |
In Pipeline https://clinicaltrials.gov/study/NCT03401398
|
|
THE SHIPPS STUDY A multi-institutional, prospective, controlled, randomized, double-blinded interventional trial to examine the potential benefits and risks of adjunctive hydrocortisone in Paediatric Septic shock. |
 |
In Pipeline https://clinicaltrials.gov/study/NCT03401398
|
|
BENITO Randomized, Multicenter, Multinational, Double-Blind Study to Compare the Pharmacokinetics, Efficacy, Safety and Immunogenicity of MB12 (Proposed Pembrolizumab Biosimilar) versus Keytruda® in Combination with Chemotherapy for the Treatment of Patients with Advanced Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) (BENITO Study) |
In Pipeline |
|
|
ATEA An Evaluation of Bemnifosbuvir-Ruzasvir (BEM/RZR) Versus Sofosbuvir-Velpatasvir (SOF/VEL) for the Treatment of Chronic Hepatitis C Virus (HCV) Infection in a Phase 3 Randomized, Controlled, Open-label Study” |
 |
In Pipeline https://clinicaltrials.gov/study/NCT06868264?cond=Hepatitis%20C&aggFilters=funderType:industry,phase:3,status:rec,studyType:int&rank=2 |
Collaborators
A Gateway to Progress in Cancer Research
The Shifa Cancer Registry (SCR) is a hospital-based cancer registry initiated in 2018 to systematically collect accurate and comprehensive data on patients diagnosed and/or treated for cancer at Shifa International Hospital (SIH). It serves as a centralized database for the collection, storage, analysis, and interpretation of cancer-related data, ensuring thorough documentation of all cancer cases at SIH.
More Than Just Statistics
A cancer registry is not just a collection of statistics—it is a dynamic resource that plays a pivotal role in advancing cancer care. By sharing comprehensive, anonymized cancer data, the registry empowers researchers, clinicians, and public health professionals to drive innovation, optimize treatments, and develop effective prevention strategies.
Work Flow
Manuals
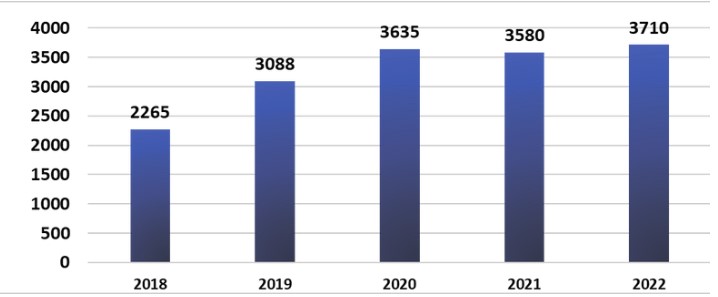
Growth in Cancer Cases (2018–2022)
The Shifa Cancer Registry recorded a 63.8% increase in cancer cases from 2018 to 2022
Together, we turn data into discovery, insight into impact, and hope into reality
Useful Links
Guidelines for Synopsis and Dissertation Writing for CPSP
National Institute of Health http://www.clinicaltrials.gov
Good Clinical Practices https://gcp.nidatraining.org/
Introduction to the Principles and Practice of Clinical Research
“Innovation, dedication, and expertise define CTU. Meet the passionate professionals who drive our mission forward every day.”
Click to Know More About Our Team


A special mention of SCRC team in the newsletter and praise from Dr. David Conen, Principal Investigator from Canada
Meeting with PJ Devereaux, principal scientist at PHRI, Canada at SIHL to discuss the HIP-ATTACK2 and ASPIRE-AF trials
Participation of the SCRC team in an expert consultation organized by the World Health Organization (WHO), NHSRC, and DRAP.
SCRC team at 17th ANRD with Dr. Fahad being the organizer and Dr. Qasim being the moderator
Meeting with Dr. Timo Tolppa, a UK-based global health researcher and coordinator of PPIE
SCRC team at Joint Commission International (JCI) reaccreditation survey
Meeting with NUST & PHC Global to review AI SAROSH progress and explore future collaboration.
SCRC team at the Symposium on Future of Clinical Trials in Pakistan at Rehman Medical Institute (RMI), Peshawar
Poster presentation by Dr. Sabahat and Dr. Sofiaat at the 14th International Public Health Conference, HSA, Islamabad.
Mr. Abdul Manan Kiana won 3rd prize in the 17th ANRD poster competition.
Certificate Distribution for the first Pharmacy Residents’ Research Course
Meeting of SCRC team for process improvements and workflow development